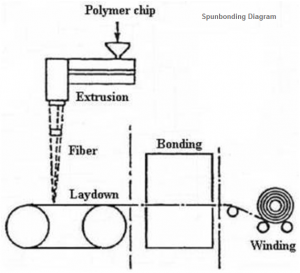
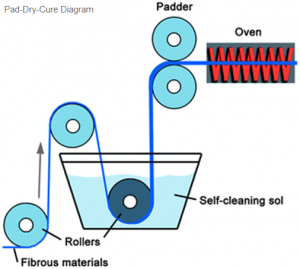
What are types of fabrics used for Personal Protective Clothing (PPE)?
Personal protective equipment (PPE) refers to protective clothing, helmets, gloves, face shields, goggles, facemasks and/or respirators or other equipment designed to protect the wearer from injury or the spread of infection or illness.
We might have few questions frequently in mind in this Corona-virus pandemic situation.
What Protective Clothing Is Appropriate for Corona-virus (2019-nCoV) Disease Control?
What quality fabric is required for making protective clothing for Novel Corona-virus Disease Control?
What are the standard requirements for Protective Clothing?
What are the fabric specifications of Protective Clothing?
Why can’t we produce fabric for protective clothing in Bangladesh to support the healthcare professional?
Is Bangladesh is capable of producing protective clothing fabric?
Which quality Protective clothing should use for the protection from Corona-virus in which condition?
Everyone should have knowledge regarding above two questions particularly in this Novel Corona-virus (2019-nCoV) pandemic situation worldwide. Otherwise we might use protective clothing to save ourselves from Corona-virus but at the end, it will not protect us from getting infected. Especially for the healthcare professionals (Doctors, Nurses & others related to medical treatment) who are the front line fighter against Corona virus. We should identify which quality standard of PPE before using it in certain condition. We should check the PPE standard before buying and using for specific reason. We should identify which level of protection will be given by the PPE.
What are the Standards of Personal Protective Clothing (PPE)?
Normally it is mentioned in the PPE tag where the manufacturer mentioned the standard level either FDA/CE/ISO standard. Let’s discuss about the standard first.
FDA (Food and Drug administration) and CE (European Commission) have their own PPE standards and need prior approval before production of those PPE. If we use wrong PPE in high risk area then very high chance of getting infected rather getting protection.
For Gown, the FDA standard describes the barrier protection levels of gowns and other protective apparel intended for use in health care facilities and specifies test methods and performance results necessary to verify and validate that the gown provides the newly defined levels of protection:
Regardless of how the product is named (that is, isolation gown, procedure gown, or cover gown), when choosing gowns, look for product labeling that describes an intended use with the desired level of protection based on the above risk levels. Product names are not standardized.
The critical protective zones for surgical and non-surgical gowns are defined differently by the standard. While the critical zones designate different protective areas for the different gowns, the levels of protection are the same for both surgical and non-surgical gowns. Liquid barrier performance is not related to the strength of the material. This standard references several other standards.
We will discuss only on gown grading base on their level on protection based on FDA and CE standard. For Novel Corona-virus protection we can follow the below instruction according to level grade from FDA. We will discuss European standard later on.
FDA Classifies a gown’s ability to act as a barrier to penetration by liquids or liquid-borne pathogens based on four levels.
PPE Grade: Level 1
Description:
- Used for MINIMAL risk situations
- Provides a slight barrier to small amounts of fluid penetration
- Single test of water impacting the surface of the gown material is conducted to assess barrier protection performance.
Applicability:
Basic care, standard hospital medical unit
PPE Grade: Level 2
Description:
- Used in LOW risk situations
- Provides a barrier to larger amounts of fluid penetration through splatter and some fluid exposure through soaking
- Two tests are conducted to assess barrier protection performance:
- Water impacting the surface of the gown material
- Pressurizing the material
Applicability:
Blood draw from a vein, Suturing, Intensive care unit, Pathology lab
PPE Grade: Level 3
Description:
- Used in MODERATE risk situations
- Provides a barrier to larger amounts of fluid penetration through splatter and more fluid exposure through soaking than Level 2
- Two tests are conducted to test barrier protection performance:
- Water impacting the surface of the gown material
- Pressurizing the material
Applicability:
Arterial blood draw, Inserting an IV, Emergency Room, Trauma
PPE Grade: Level 4
Description:
- Used in HIGH risk situations
- Prevents all fluid penetration for up to 1 hour
- May prevent VIRUS penetration for up to 1 hour
- In addition to the other tests conducted under levels 1-3, barrier level performance is tested with a simulated blood containing a virus. If no virus is found at the end of the test, the gown passes.
Applicability:
Pathogen resistance, Infectious diseases (non-airborne), Large amounts of fluid exposure over long periods
FDA recommends below for using gowns for Corona-virus protection:
- Implementing the use of reusable gowns instead of disposable single use gowns.
- Using ANSI/AAMI PB70 standard Level 3 or 4 gown (that is, sterile surgical isolation gowns) for surgery/invasive procedures with a medium to high risk of contamination.
- Isolation gowns for routine care of patients that are suspected or confirmed to be infected with COVID-19.
The European Union issued a number of regulations to improve health and safety at work and to ensure high quality PPE. The PPE Regulation (EU) 2016/425 covers the manufacturing and marketing of personal protective equipment. It defines legal obligations to ensure that PPE on the European market provides the highest level of protection against hazards. The CE marking affixed to PPE provides evidence of this protection.
As this is a “New Approach” Directive, manufacturers or their authorised representative in the EU can comply with the technical requirements directly or with European Harmonised Standards. The latter provides a presumption of conformity to the essential health and safety requirements.
As per CE standard, there are three categories of PPE as explained below. If you see the mentioned protective clothing having approval under category III, then only we can consider using for Corona-virus disease control.
Let’s have a brief of all the three categories…
Category I :
PPE intended to protect users against minimal risks. Category I includes exclusively PPE
intended to protect users against the following risks:
(a) superficial mechanical injury;
(b) contact with water or cleaning materials of weak action;
(c) contact with hot surfaces not exceeding 50°C;
(d) damage to the eyes due to exposure to sunlight (other than during observation
of the sun);
(e) atmospheric conditions that are not of an extreme nature.
Category II :
Category II includes:
(a) PPE intended to protect users against risks other than those listed in Categories
I and III;
(b) made-to-measure PPE except where such PPE is intended to protect users
against risks listed in Category I.
Category III :
PPE intended to protect users against very serious risks. Category III includes exclusively
PPE intended to protect users against the following risks:
(a) inhalation of harmful substances;
(b) aggressive chemicals;
(c) ionising radiation;
(d) high-temperature environments the effects of which are comparable to those of
an air temperature of at least 100°C;
(e) low-temperature environments the effects of which are comparable to those of
an air temperature of -50°C or less;
(f) falling from a height;
(g) electric shock and live working;
(h) drowning;
(i) cuts by hand-held chain-saws;
(j) high-pressure cutting;
(k) bullet wounds or knife stabs;
(l) harmful noise.
Producers must have to take prior marking approval before selling of protective clothing from CE as per standard procedure in EU market.
There are few standards of protective clothing according to their area of use and level of protection given….
In order to avoid misappropriate Personal Protective Equipment (PPE) during this corona-virus pandemic battle, we need to use protective clothing under category III and EN 14126:2003 standard clothing, which is the standard specifies a set of requirements and test methods to measure the fabric protection against infective agents.
EN 14126:2003 includes 5 test methods to determine the protection class against several specific biological hazards listed as the following:
ISO 16603:
Resistance to penetration by blood/body fluids.
ISO 16604:
Resistance to penetration by blood borne pathogens
ISO 22610:
Resistance to wet microbial penetration
ISO/DIS 22611:
Resistance to liquid aerosol penetration
ISO 22612:
Resistance to dry microbial penetration
COVID-19 belongs to the Corona-viruses family, with size of approximately 0.125 microns. From the above contaminants, Phi-X-174 (0.027 microns) is the only contaminant which smaller than COVID-19. Hence, if the protective clothing passes ISO 16604 with a relatively high protection class, it means that it has a higher protection level.
If you are frontline medical personnel, you might focus on ISO/DIS 22611. Earlier in February, China admitted that aerosol transmission is possible. It could happen when the patient sneezes hard or when you are exposed to high aerosol concentrations in an enclosed environment for a long time, such as inserting respiratory tube during medical procedures, which causes a burst of mass aerosols.
In a conclusion, during the selection of protective clothing against COVID-19, you can first identify EN 14126:2003 certification via wording “-B” behind “TYPE” classification, namely “TYPE 3-B”, “TYPE 4-B” and “TYPE 5-B” in product series. Once identified the protection capability against infective agents, do take a closer look at respective test results according to your job specification in particular working environment.
Hope everyone is clear about quality standard and parameters that needs to check before using protective clothing. Hope everyone is aware of appropriate protective selection and parameters checkpoints.
What are the best fabric Construction for the PPE ?